Cations are positively charged ions that form when atoms lose electrons. When an atom loses electrons, it results in more protons than electrons, giving the atom an overall positive charge. Cations play a key role in chemistry because of their tendency to form ionic bonds with negatively charged ions, known as anions. The interactions between cations and anions are responsible for the formation of ionic compounds like salts. Cations also influence the pH and acidity of solutions. Understanding how cations form and behave is therefore fundamental to many areas of chemistry.
Atomic Structure
Atoms consist of protons, neutrons, and electrons. Protons have a positive charge, neutrons have no charge, and electrons have a negative charge. The number of protons in an atom determines its atomic number and identity as an element. For example, all atoms with 6 protons are atoms of the element carbon. Atoms are electrically neutral when they contain an equal number of protons and electrons. However, atoms can gain or lose electrons to become charged ions.
When an atom loses one or more electrons, it is left with more protons than electrons, giving it an overall positive charge. For example, a sodium atom has 11 protons and 11 electrons, making it neutral. But if it loses one electron, it will have 11 protons and only 10 electrons, leaving it with a net positive charge of +1. This positively charged ion is called a cation. The charge comes from the imbalance between the number of protons and electrons. Any atom that loses electrons to form a cation will have more protons than electrons, giving it that positive charge.
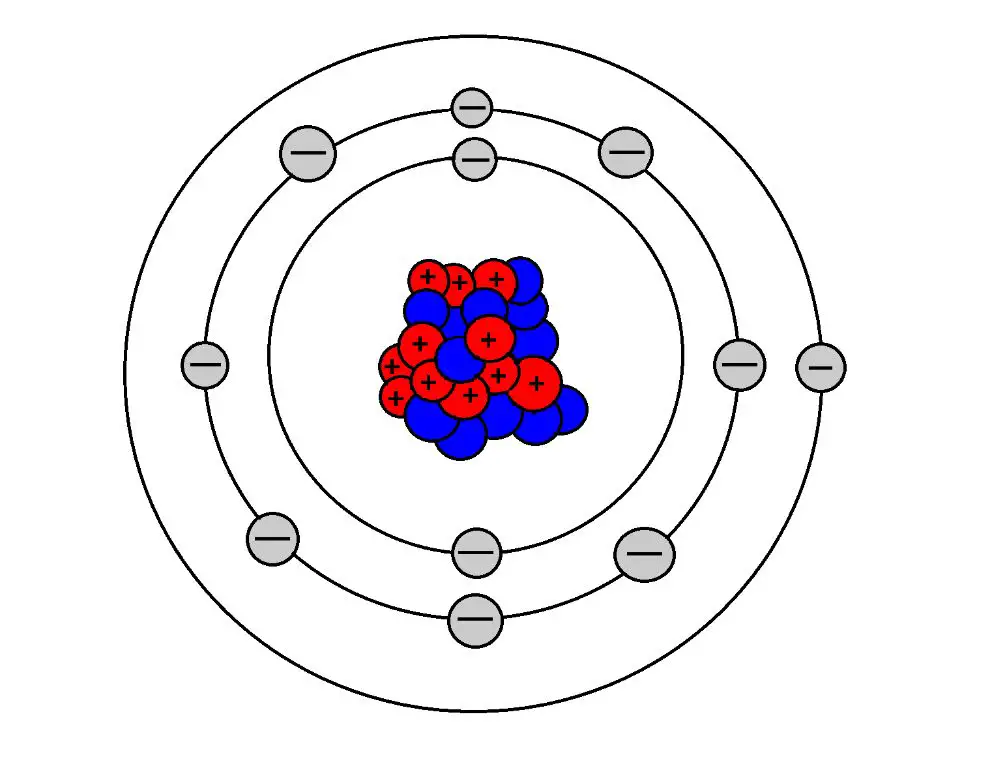
According to LibreTexts, the number of electrons lost determines the charge on the cation. For example, if a sodium atom loses two electrons instead of one, it will have a +2 charge because its 11 protons now outweigh the 10 remaining electrons.
Ionization
Ionization is the process by which atoms gain or lose electrons to become charged particles called ions. Atoms that lose electrons become positively charged cations (https://energyeducation.ca/encyclopedia/Ionization, https://chem.libretexts.org/Courses/University_of_Kentucky/UK%3A_CHE_103_-_Chemistry_for_Allied_Health_(Soult)/Chapters/Chapter_2%3A_Elements_and_Ions/2.5%3A_Ion_Formation). When an atom loses one or more electrons, it becomes positively charged because it now has more protons than electrons. The charge of a cation is equal to the number of electrons lost.
For example, a sodium atom has 11 protons and 11 electrons, making it neutral. If it loses one electron, it now has 11 protons and only 10 electrons, giving it a +1 charge. It has become a positively charged sodium ion or sodium cation. Atoms can lose multiple electrons to form cations with charges of +2, +3 etc, depending on the number of electrons lost (https://chem.libretexts.org/Bookshelves/Introductory_Chemistry/Book%3A_Introductory_Chemistry_(CK-12)/08%3A_Ionic_and_Metallic_Bonding/8.03%3A_Cation_Formation).
The process of losing electrons to become cations is called ionization. It occurs through various mechanisms like metal oxidation, acid reactions, combustion, etc. The resulting cations are key components that interact to form ionic compounds.
Metals Forming Cations
Metals tend to lose electrons and form positively charged cations. This happens because metals have relatively low ionization energies and electronegativity values. When a metal atom loses one or more of its valence electrons, it becomes a positively charged ion known as a cation.
For example, sodium (Na) has a low first ionization energy of 495.8 kJ/mol. If sodium loses one of its valence electrons, it forms the Na+ cation. The sodium cation has a charge of +1, indicating it has lost a single electron. Other metals like magnesium, aluminum, and calcium also readily form +1 or +2 cations by losing valence electrons.
Transition metals like zinc, iron, copper, and nickel have two valence electrons in their outermost energy level. When transition metals lose these electrons, common transition metal cations like Zn2+, Fe2+, Cu2+, and Ni2+ are formed. Therefore, metals form cations through the loss of valence electrons, resulting in a positive charge.
Source: Chemistry Exam 1 Review
Acid Solutions

When acids react with metals, the metals give up one or more electrons to form positively charged ions called cations. The loss of electrons is called oxidation, so metal atoms are oxidized when they react with acids.
For example, zinc metal reacts with hydrochloric acid to produce zinc chloride and hydrogen gas. The reaction is:
Zn(s) + 2HCl(aq) → ZnCl2(aq) + H2(g)
In this reaction, the zinc atom loses two electrons to become a Zn2+ cation. The hydrogen ions (H+) from the acid accept the electrons to form hydrogen gas (H2).
Magnesium also readily reacts with acids to form magnesium cations. The reaction between magnesium metal and sulfuric acid is:
Mg(s) + H2SO4(aq) → MgSO4(aq) + H2(g)
Here, the magnesium atom loses two electrons to form Mg2+. The hydrogen ions in the acid accept the electrons, forming hydrogen gas as a product.
Acids provide an abundance of hydrogen ions that can accept electrons from metals. This oxidation process allows metals like zinc and magnesium to form positively charged cations when reacted with acids.
Combustion Reactions
Combustion reactions involve the rapid reaction of a fuel with an oxidizer, usually oxygen, to release energy in the form of heat and light. Many combustion reactions result in cation formation as the fuel, often a metal, loses electrons and becomes oxidized.
A classic example of a combustion reaction that forms a cation is the burning of sodium metal in oxygen to form sodium oxide:

2Na(s) + O2(g) → Na2O(s)
In this reaction, each sodium atom loses one electron to become a Na+ cation. The oxidation state of sodium goes from 0 in Na(s) to +1 in Na2O(s).
Another common example is the combustion of magnesium metal:
2Mg(s) + O2(g) → 2MgO(s)
Here, magnesium atoms are oxidized from 0 to +2 oxidation state, forming Mg2+ cations in the product magnesium oxide.
Most alkali metals and alkaline earth metals readily form cations through combustion reactions with oxygen. The electrons lost in the oxidation reaction are transferred to oxygen, reducing it to oxide ions (O2-).
Oxidation Reactions
Oxidation reactions involve the loss of electrons by an atom, molecule or ion. This loss of electrons results in an increase in oxidation state and often leads to the formation of cations. Cations are positively charged ions that form when an atom loses one or more valence electrons.
A common example of an oxidation reaction that forms cations is the reaction of a metal with oxygen. For instance, when sodium metal reacts with oxygen gas, the sodium atoms lose electrons and become sodium ions (Na+). The oxidation reaction is:
2Na(s) + O2(g) → 2Na+ + O2-
Here, the sodium atoms are oxidized, losing one electron each to form the Na+ cation. The oxygen gains electrons from the sodium and is reduced to the oxide (O2-) anion. This demonstrates how oxidation leads to cation formation in metals.
Another example is the oxidation of iron metal to form iron cations:
2Fe(s) + 3O2(g) → 2Fe3+ + 3O2-
In this reaction, the iron atoms are oxidized from the Fe0 state to Fe3+, losing three electrons each and forming iron cations. Oxidation reactions like these are common ways that cations form when metals react.
(Conducting Polymers for Advanced Energy Applications. https://books.google.com/books?id=2dJKEAAAQBAJ&pg=RA1-PA76&lpg=RA1-PA76&dq=%22oxidation+reactions+that+produce+cations%22&source=bl&ots=L-ZkbcYIBJ&sig=ACfU3U1AgkGq2MyD_Ut2Ze8hy034ti1M6w&hl=en&sa=X&ved=2ahUKEwjUs4LJmrKDAxVJLUQIHTTeBM8Q6AF6BAgTEAM)
Applications
Cations have a variety of important applications in fields such as medicine, industry, and energy storage.
In medicine, cationic drugs can be used for their antimicrobial properties. Many disinfectants and antiseptics are cationic, such as chlorhexidine which is used for surgical scrubs and hand washing. Cationic drugs can also facilitate the delivery of nucleotides into cells for gene therapy applications [1].
In industry, cation exchange is utilized for water treatment. Cation exchange resins exchange harmless cations like sodium for hazardous heavy metal cations like lead and mercury, removing them from water. This makes the water safe to use [2].
Cations are also critical in batteries and energy storage. Lithium ion batteries contain lithium cations which move between the anode and cathode as the battery charges and discharges. The flow of lithium cations provides the electrical current. Other metal cations like sodium, magnesium, and aluminum are also being investigated for battery applications [3].
Overall, cations play integral roles in medicine, industry, batteries, and other technologies due to their unique chemical properties and interactions.
Summary
In summary, cations are formed when atoms lose electrons and become positively charged ions. This commonly happens through three main processes:
– Metals tend to give up electrons easily to become cations. For example, sodium can lose an electron to form the Na+ cation.
– In acid solutions, hydrogen ions (H+) are produced when acids ionize in water. H+ is a common cation.
– During oxidation reactions, atoms give up electrons to oxidizing agents and become cations. For instance, iron Fe can oxidize to Fe2+ or Fe3+.
Cations are abundant in nature and important for various chemical processes and applications. Understanding how they form helps chemists control cation formation for desired outcomes.
Further Reading
If you want to learn more about how cations form, check out these additional resources:
- Khan Academy: Cation Formation – A helpful video explaining the process of forming cations in more detail.
- Boundless Chemistry: Formation of Ions – An in-depth written overview of how both cations and anions form during ionic bonding.
- ACS ChemMatters: The Chemistry Behind the Tears from Peeling Onions and Potatoes – An article explaining how acid-base reactions form cations when peeling certain vegetables.
- Lumen Learning: The Formation of Ions – A chapter from an online chemistry textbook digging into ion formation and providing examples.
These resources offer more details, visuals, examples and explanations to supplement this article’s overview of how cations form. Please explore them if you are looking to deepen your understanding of cation formation.

