Cations and anions are ions – atoms or molecules that have a positive or negative charge due to gaining or losing electrons. Understanding how cations and anions form is important in chemistry because it allows us to predict the charge of ions and understand the properties of ionic compounds.
A cation is a positively charged ion that forms when an atom loses one or more valence electrons, leaving it with a positive charge. Common cations include metals like sodium, calcium, and potassium.
An anion is a negatively charged ion that forms when an atom gains one or more electrons, giving it a negative charge. Common anions include nonmetals like chloride, bromide, and oxide.
Knowing how ions form allows us to determine their charges and write proper chemical formulas. It also allows us to understand the structure and bonding in ionic compounds. Mastering cation and anion formation is a key foundation for many areas of chemistry.
Atomic Structure
Atoms are made up of three main subatomic particles: protons, neutrons, and electrons (Quizlet). The nucleus of the atom contains protons and neutrons. Protons have a positive charge and neutrons have no charge. Electrons orbit the nucleus and have a negative charge.
The atomic number of an element refers to the number of protons in the nucleus. This number is unique for each element. The mass number refers to the total number of protons and neutrons in the nucleus. Atoms of the same element can have different mass numbers depending on the number of neutrons.
Electrons occupy different energy levels or shells around the nucleus. The innermost shell can hold up to two electrons, the second shell can hold up to eight electrons, and outer shells can hold even more. The electron configuration describes the filling of the energy levels with electrons.
Ionic Bonding
Ionic bonds are formed between metal atoms that lose electrons and nonmetal atoms that gain electrons, resulting in charged particles known as cations (positively charged) and anions (negatively charged).
In an ionic bond, the metal atom donates one or more valence electrons to the nonmetal atom. This electron transfer allows both atoms to achieve a stable octet configuration. The metal atom loses electrons and becomes a positively charged cation, while the nonmetal atom gains electrons and becomes a negatively charged anion.
For example, when sodium (Na) reacts with chlorine (Cl), the sodium atom donates its single valence electron to the chlorine atom. The sodium atom now has 11 protons (11+) and 10 electrons (10-), making it a positively charged cation (Na+). The chlorine atom now has 17 protons (17+) and 18 electrons (18-), making it a negatively charged anion (Cl-). The resulting ionic compound is sodium chloride (NaCl).
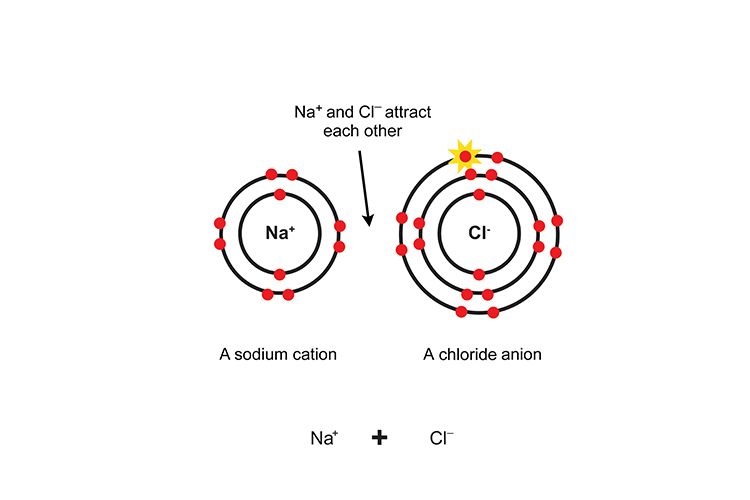
The attraction between the positively charged cations and negatively charged anions forms a stable ionic bond. Ionic compounds arrange into crystal lattice structures that maximize these electrostatic interactions between oppositely charged ions. The exchange of electrons allows both atoms to achieve a stable electron configuration and form the electrostatic attractions of an ionic bond.
Source: OpenStax Chemistry – Ionic Bonding
Cation Formation
Cations are positively charged ions that are formed when atoms lose electrons. This loss of electrons leaves the atom with more protons than electrons, creating a positive charge. Metals tend to form cations readily when bonding with nonmetals to make ionic compounds. For example, sodium (Na) has 11 protons and 11 electrons when neutral. But it can lose one electron to become a Na+ cation with 11 protons and only 10 electrons, giving it an overall +1 charge. Similarly, magnesium (Mg) has 12 protons and 12 electrons when neutral. It can lose two electrons to become a Mg2+ cation with 12 protons and only 10 electrons, giving it a +2 charge.
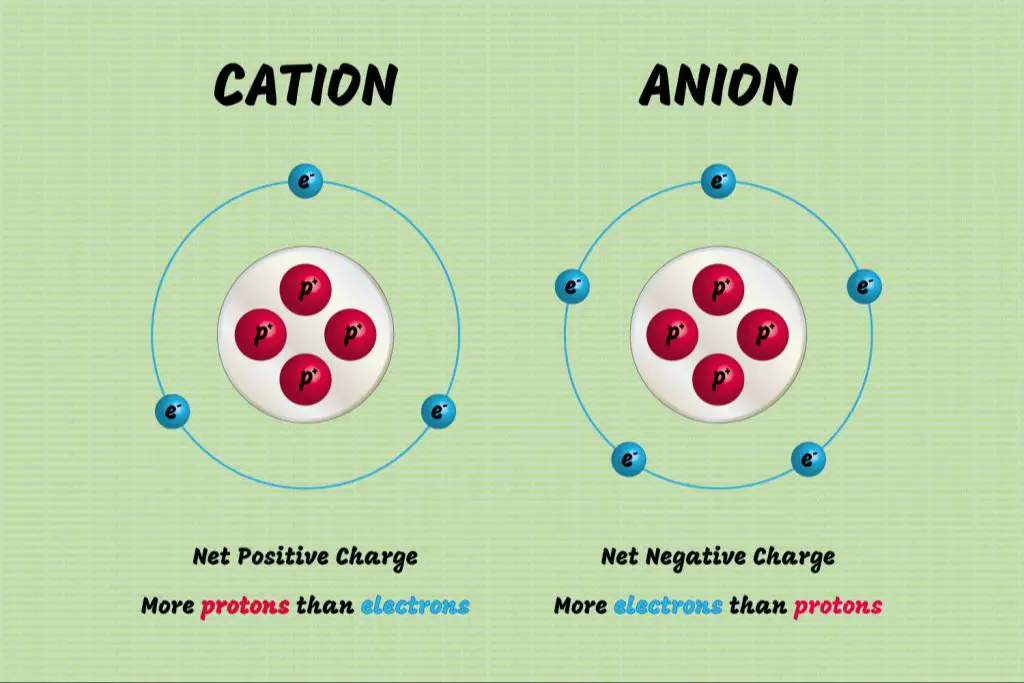
Examples of common cations formed by losing electrons include:
- Sodium ion (Na+): Sodium loses one electron to form a +1 cation
- Magnesium ion (Mg2+): Magnesium loses two electrons to form a +2 cation
- Aluminum ion (Al3+): Aluminum loses three electrons to form a +3 cation
- Iron ion (Fe2+ or Fe3+): Iron can lose two or three electrons to form +2 or +3 cations
In summary, cations are formed when atoms lose electrons, resulting in fewer electrons than protons and an overall positive charge. Metals tend to lose electrons readily to nonmetals, forming positively charged cations in the process.
Anion Formation
Anions are formed when atoms gain electrons, resulting in a negative charge. This occurs because the number of electrons is greater than the number of protons in the atom. When an atom gains one or more electrons, it becomes a negatively charged ion known as an anion.
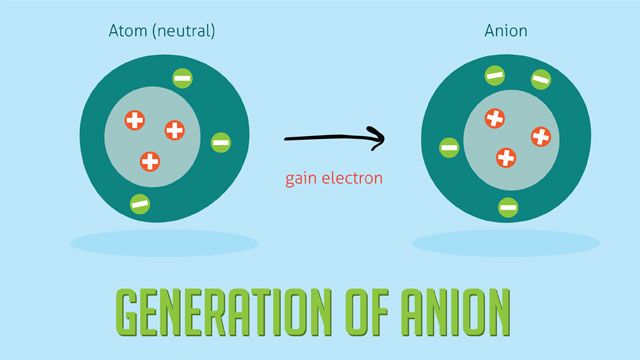
Some common examples of anion formation include:
- Chloride ion (Cl-): A chlorine atom has 7 electrons naturally. When it gains 1 electron, it becomes chloride ion with 8 electrons, giving it a negative charge.
- Oxide ion (O2-): An oxygen atom has 8 electrons naturally. When it gains 2 electrons, it becomes an oxide ion with 10 electrons, giving it a negative charge.
The gain of electrons and resulting negative charge is what defines an anion. Atoms that tend to gain electrons easily include halogens like chlorine, and oxygen group elements. The periodic table can be used to identify atoms that readily form anions.
Naming Cations
Cations are named based on their charge and the name of the element. For metals that form only one cation, the name is just the element name followed by the cation charge in Roman numerals in parentheses. Examples include lithium (Li+), sodium (Na+), potassium (K+), calcium (Ca2+), iron (Fe2+ or Fe3+), copper (Cu+ or Cu2+), zinc (Zn2+), and magnesium (Mg2+).
For metals that can form multiple cations with different charges, Roman numerals indicating the charge are appended to the element name. Examples include iron (Fe2+ or Fe3+), copper (Cu+ or Cu2+), tin (Sn2+ or Sn4+), and lead (Pb2+ or Pb4+). The higher charge is indicated with a higher numeral.
Transition metals can form multiple cations so the charge is indicated using Roman numerals in parentheses after the element name. Common transition metal cations include chromium (Cr3+), manganese (Mn2+), iron (Fe2+, Fe3+), cobalt (Co2+, Co3+), nickel (Ni2+), copper (Cu+ or Cu2+), and zinc (Zn2+).
According to the source Naming ions and ionic compounds, these conventions allow chemists to determine the charge and element identity for each cation based on its name.
Naming Anions
Anions are named based on the charge and element name. Monoatomic anions contain a single element and use the following naming conventions:
- If the element forms only one negative ion, add -ide to the end of the element name (e.g. Cl- becomes chloride)
- If the element forms multiple ions, the charge is written in parentheses after the element name (e.g. O2- becomes oxide)
Some examples of monoatomic anion names are chloride (Cl-), oxide (O2-), sulfide (S2-), and nitride (N3-).
Polyatomic anions contain multiple elements and usually have special names. Some examples are hydroxide (OH-), acetate (CH3COO-), carbonate (CO32-), and phosphate (PO43−). The charges are not specified when naming polyatomic anions.
To summarize, both monoatomic and polyatomic anions are named based on the elements present and charge when applicable. Special naming conventions exist to easily identify the ions based on these properties.
Source: https://quizlet.com/421024191/chem-135-umd-griffith-flash-cards/
Writing Ionic Formulas
Ionic compounds are formed between metal cations and nonmetal anions. To write the proper chemical formula, we need to consider the charges on the ions. Here are the steps to writing formulas for ionic compounds:
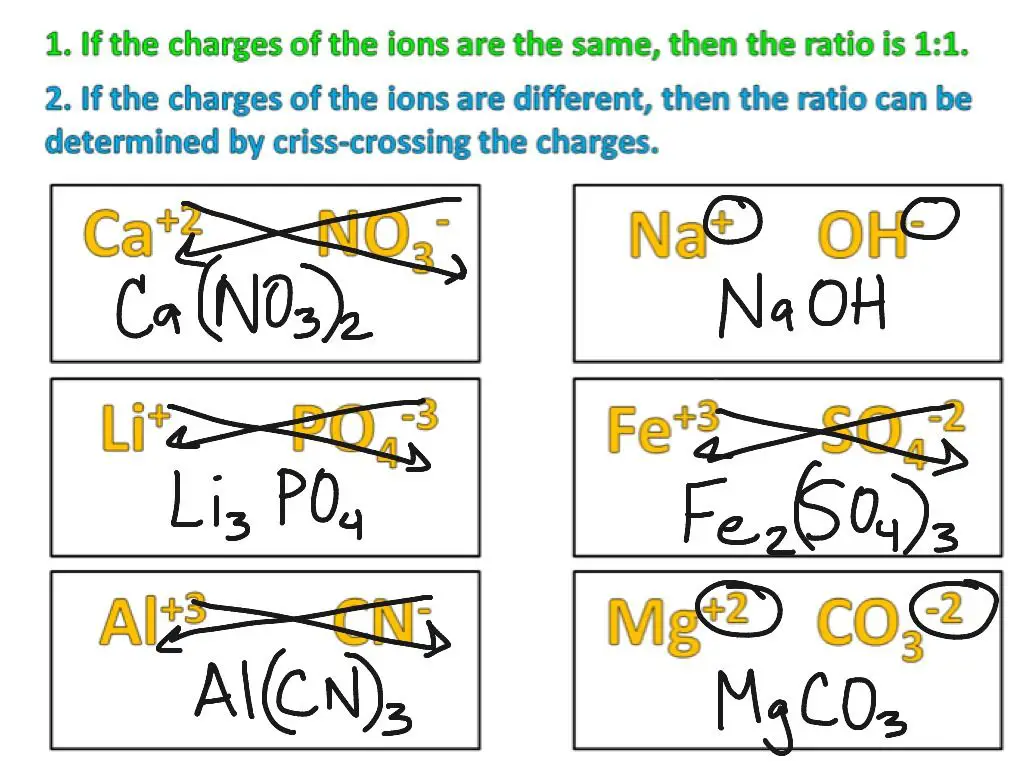
1. Identify the cation and anion in the compound. Write the symbol and charge for each.
2. Determine the simplest whole number ratio between the charges that will result in a neutral compound. This will tell you the subscripts for each ion in the formula.
3. Write the cation first followed by the anion. Include the subscripts if greater than 1.
For example, to write the formula for lithium fluoride:
1. The cation is Li+ and the anion is F-.
2. To balance the +1 charge on lithium and the -1 charge on fluoride, we need a 1:1 ratio.
3. Writing the cation first, the formula is LiF.
As another example, for magnesium chloride:
1. The cation is Mg2+ and the anion is Cl-.
2. To balance the +2 charge on magnesium and the -1 charge on chloride, we need a 1:2 ratio.
3. The formula is MgCl2.
The charges dictate the proper chemical formula for ionic compounds. Understanding the steps allows you to systematically write the correct formula based on the ions present. For more examples, see Writing Formulas for Ionic Compounds.
Real-World Applications
Cations and anions play critical roles in biology, industry, and technology. In the human body, potassium cations (K+) and sodium cations (Na+) are essential for nerve impulse transmission, muscle contraction, and maintaining fluid balance (https://study.com/academy/lesson/cations-definition-examples.html). Calcium cations (Ca2+) are needed for healthy bones and teeth. Anions like chloride (Cl-), bicarbonate (HCO3-), and phosphate (PO43-) help regulate pH and fluid levels in the body.
In industry, ion exchange resins containing cations like sodium (Na+) and anions like hydroxide (OH-) are used to soften hard water by removing calcium (Ca2+) and magnesium (Mg2+) ions. Cation and anion exchange resins also have applications in wastewater treatment, hydrometallurgy, chromatography, and nuclear power (http://www.scienceclarified.com/everyday/Real-Life-Chemistry-Vol-1/Ions-and-Ionization-Real-life-applications.html).
Many common household chemicals like table salt (NaCl), baking soda (NaHCO3), bleach (NaClO), and detergents contain ions that impart important properties. Salts like NaCl and MgCl2 are used extensively for deicing roads in winter. Ionic compounds also have key uses in agriculture as fertilizers and food additives.
Conclusion
In summary, anions and cations form due to electrons being transferred between atoms. Atoms that lose electrons become positively charged cations, while atoms that gain electrons become negatively charged anions. The formation of cations and anions allows atoms to achieve stable electron configurations and is the basis for ionic bonding. Understanding cation and anion formation is fundamental to chemistry, as it explains the behavior of atoms and compounds.
This article provided an overview of atomic structure, ionic bonding, the processes behind cation and anion formation, naming conventions, writing ionic formulas, and real-world applications. The key takeaways are that cations form when atoms lose electrons, anions form when atoms gain electrons, and the resulting charges allow ions to electrostatically attract and form ionic compounds. Mastering cation and anion concepts provides a strong foundation for further chemistry learning and insight into the behavior of matter at the atomic scale.

