What are Cation Exchange Resins?
Cation exchange resins are insoluble polymers that contain negatively charged functional groups. They are able to exchange their bound cations for others in a solution [1]. The cation exchange process involves the reversible interchange of cations between the solid resin phase and the liquid phase.
Cation exchange resins were first developed in the 1930s by Adams and Holmes. They created phenol-formaldehyde resins that could exchange cations. Since then, synthetic ion exchange resins have been widely used for water treatment, metals recovery, medicine production, and various analytical purposes [2].
Common applications of cation exchange resins include water softening, demineralization, metals removal, buffering solutions, catalysis, and drug formulation. They are often used to remove harmful cations and purify water while adding beneficial cations like sodium or potassium [3].
Cation Exchange Resin Chemistry
Cation exchange resins are made up of an insoluble matrix or support structure, usually an organic polymer or mineral, that contains negatively charged functional groups. These functional groups provide binding sites that attract and exchange positively charged cations from the surrounding solution. The most common functional groups in cation exchange resins are sulfonic acid groups (-SO3H) and carboxylic acid groups (-COOH) [1].
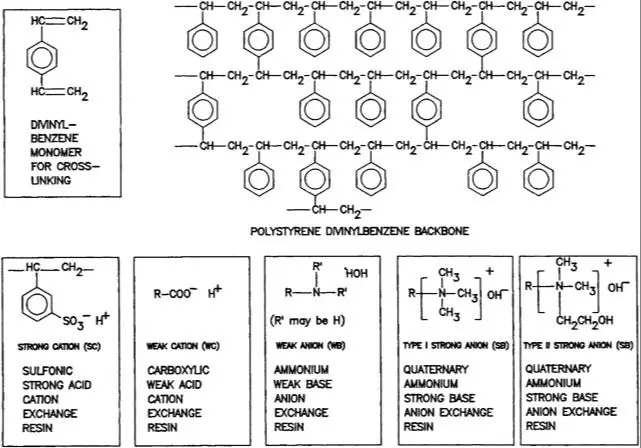
In contrast, anion exchange resins contain positively charged functional groups like quaternary ammonium (-NR3+) that attract and bind negatively charged anions. This difference in charged functional groups is what distinguishes cation exchange resins from anion exchange resins [2]. While cation resins exchange positively charged ions, anion resins exchange negatively charged ions.
The support matrix for both cation and anion exchange resins is often made from polystyrene cross-linked with divinylbenzene. The cross-linking provides insolubility and mechanical stability. Other common matrix materials include cellulose, dextran, agarose, polyacrylamide, and inorganic minerals.
[1] https://www.britannica.com/science/cation-exchange-resin
[2] https://en.wikipedia.org/wiki/Ion-exchange_resin
How Cation Exchange Works
Cation exchange resins are cross-linked polymers with negatively charged functional groups attached to the polymer matrix. These groups are fixed and cannot move. While the resin is immobile, ions in solution can move freely. When a solution containing cations (positively charged ions) comes in contact with the resin, the cations are attracted to the negatively charged functional groups. This attraction is an ionic interaction based on opposite charges. The cations exchange with previously bound counter-ions on the resin.
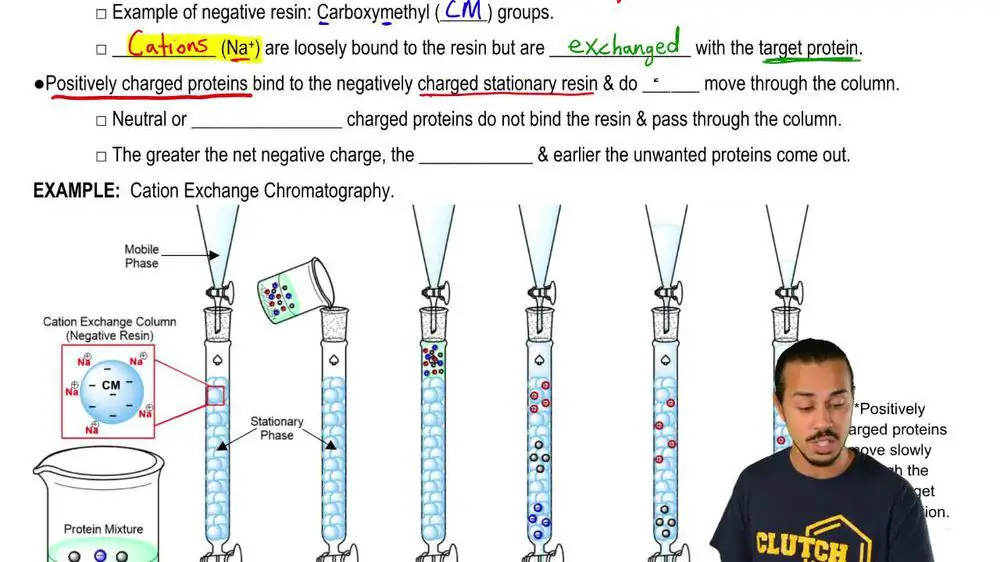
The ion exchange process involves two simultaneous actions – the counter-ion previously bound is released into solution and the ion in solution is bound to the exchange site. The affinity of the resin for different cations varies based on properties of the ions such as charge, size, and electronegativity. Monovalent ions like Na+ are weakly held while multivalent ions like Ca2+ are strongly attracted.
Eventually the resin becomes saturated with ions and must be regenerated to continue exchanging ions. Regeneration uses a high concentration solution to replace the adsorbed ions with ions that are preferable for the intended application. For cation exchange resins, regeneration typically uses an acid like hydrochloric acid or sulfuric acid to replace adsorbed cations with hydrogen ions, restoring the resin to the hydrogen form.
Types of Cation Resins
Cation exchange resins are categorized based on their ion exchange properties. The two main types are strong acid cation resins and weak acid cation resins.
Strong acid cation resins contain sulfonic acid groups that enable complete ionization and rapid ion exchange kinetics. Common strong acid resins include styrene-divinylbenzene copolymers with sulfonic acid functional groups (https://www.sciencedirect.com/topics/chemistry/ion-exchange-resin).
Weak acid cation resins contain carboxylic acid groups that are only partially ionized at neutral pH. Weak acid resins offer selective ion exchange based on the pKa of the functional group. Common examples are acrylic and methacrylic resins with carboxylic acid groups.
Cation resins are also classified based on their physical structure as gel or porous types. Gel resins have a homogeneous structure, while porous resins have pores that provide access to interior ion exchange sites. Porous resins generally have higher capacity but slower kinetics compared to gel resins.
Overall, strong acid gel resins like sulfonated styrene-divinylbenzene are the most commonly used cation exchange resins due to their high capacity, structural stability, and fast kinetics. Weak acid porous resins provide more selectivity for targeted cation separations.
Cation Exchange Capacity
Cation exchange capacity (CEC) refers to the total number of exchangeable cations that a soil can adsorb at a specific pH value[1]. It is an inherent characteristic of soil that is dependent on the clay and organic matter content. Soils with higher clay and organic matter contents tend to have higher CEC. CEC influences many aspects of soil fertility since it gives an indication of the soil’s ability to hold onto essential nutrients such as potassium, calcium, and magnesium cations.
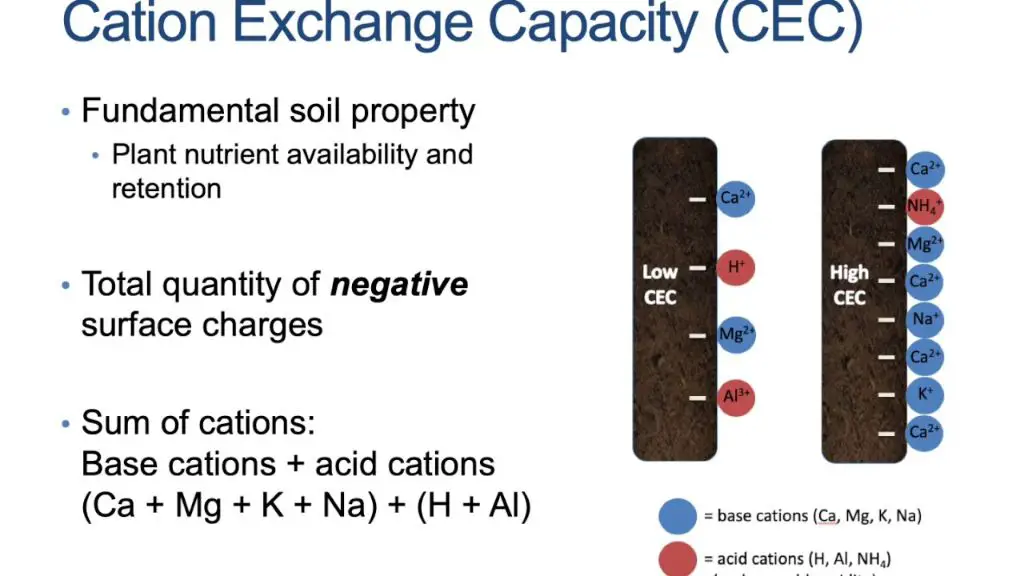
Some key factors that affect CEC include:[2]
- Clay Content – Clay particles have a net negative charge which attracts and holds positively charged nutrients.
- Organic Matter – Organic matter contains carboxylic and phenolic functional groups that contribute negative charges.
- Soil pH – The CEC declines when soil pH is very high or very low, with maximum CEC occurring in the pH range of 6.5-7.0.
- Cation Valency – Multivalent cations are held more tightly to clay and organic matter surfaces than monovalent cations.
There are several methods for measuring CEC in the lab and field. The most direct method is saturating the exchange sites with an index cation like ammonium, then measuring exchangeable ammonium using an extraction and quantification technique[3].
[1] https://www.soilquality.org.au/factsheets/cation-exchange-capacity
[2] https://www.extension.purdue.edu/extmedia/ay/ay-238.html
[3] https://www.soilquality.org.au/factsheets/cation-exchange-capacity
Applications of Cation Resins
Cation exchange resins have many important industrial applications including:
Water Treatment
One of the most common uses of cation exchange resins is in water treatment. The resins are used to soften hard water by exchanging calcium and magnesium ions for sodium ions. This prevents scale formation in pipes and improves the quality of the water (Water Technology). Cation resins can also remove toxic heavy metals like lead and arsenic from drinking water.
Food and Beverage Processing
Cation exchange resins are utilized in the food and beverage industry to improve the taste and quality of products. They can remove bitter compounds, clarify juices and wines, and adjust acidity levels (Lab Training). The resins also play a role in sugar production, helping filter and decolorize syrups.
Pharmaceutical Manufacturing
Cation exchange chromatography is widely used in pharmaceutical manufacturing to purify drug compounds. The resins can isolate active ingredients, remove impurities, and produce highly pure products. Cation resins also enable testing and quality control of drug products.
Other Industrial Uses
Other applications include purification of chemicals, recovery of precious metals, treatment of nuclear waste, and processing of electroplating waste. Cation resins play an important role across many industries by facilitating separation and purification processes.
Cation Resin Regeneration
Cation exchange resins need to be periodically regenerated to restore their ion exchange capacity and continue working effectively. The regeneration process removes the ions that have become attached to the resin during operation and replaces them with hydrogen and hydroxyl ions. Proper regeneration is crucial for achieving a high quality effluent and preventing premature exhaustion of the resin bed.

The most common way to regenerate cation resins is through chemical regeneration. This involves passing a concentrated acid, typically hydrochloric acid or sulfuric acid, through the resin bed. The acidic solution protonates the cation exchange sites, displacing the bound cations. After regeneration, the bed must be thoroughly rinsed to remove any excess regenerant.
The frequency of regeneration depends on the application. For potable water treatment, regeneration may only be needed every 1-2 weeks. For industrial processes and power applications, regeneration could be required daily or even more frequently. Monitoring the effluent quality and tracking the system’s run lengths helps determine optimal regeneration cycles. More frequent regeneration leads to higher operating costs but prevents the resin from becoming overloaded.
Overall, resin regeneration allows continuous reuse of cation exchange resins. When performed properly at suitable intervals, regeneration sustains capacity and prevents costly resin replacement.
Sources:
The Basics of Resin Regeneration
Selecting Cation Resins
There are several important considerations when selecting a cation exchange resin for an application:
The nature of the target compounds – Size, charge density, and chemical composition can help determine the ideal resin chemistry and porosity. Smaller compounds may require gels, while larger ones need macroporous resins.
Separation requirements – High resolution separations demand higher ion exchange capacity and more uniform particle sizes. Analytical separations may need very specific selectivity.
Chemical compatibility – The resin must be stable in the mobile phase and resistant to oxidation and degradation. Styrenic resins offer the best stability.
Desired binding strength – Weak acid resins provide reversible ionic binding, while strong acid resins form nearly irreversible covalent bonds. Selectivity depends on binding strength.
Regeneration method – The choice of regenerant impacts the long-term efficiency. Harsh chemicals limit options.
Cost considerations – Higher quality resins have increased capacity, chemical stability and lifespan, but also higher prices. Balance performance against budget.
Testing different resin types with real samples helps identify the optimal selectivity, capacity, and kinetics required. Pilot studies using radial flow columns can simulate the actual separation environment.
Overall, resin properties must be matched to the specific solutes, impurities, and operating conditions involved. Consulting guides like this resin selection overview can aid the selection process.
Cation Resin Safety
When handling cation exchange resins, it is important to take proper safety precautions. Cation resins are generally considered non-hazardous if handled correctly, but there are some health hazards to be aware of.
Most cation resins are based on polystyrene sulfonic acid and are supplied in the hydrogen form. The raw resins themselves are not acutely toxic, but care should be taken to avoid ingestion or inhalation of dust from the resin beads. Proper PPE like gloves, goggles, and dust masks should be used when handling dry resin to prevent exposure. Resin dust can be irritating to eyes, skin, and respiratory system (Safety Data Sheet – Ion Exchange Resin).
When wet, cation resins are not hazardous. However, when regenerating used resin, concentrated acids like hydrochloric or sulfuric acid are often used which can cause severe burns. Proper protective equipment like acid-resistant gloves, aprons, goggles and faceshields should be used when handling regeneration acids.
For disposal, cation resins can simply be landfilled as non-hazardous solid waste. However, if the resin has absorbed hazardous materials like heavy metals, it may require special handling as a hazardous waste. Spent resins should be rinsed thoroughly to remove any absorbed hazardous materials before disposal (Ion Exchange Resin Safety Data Sheet). With proper safe handling procedures, cation exchange resins present minimal health or environmental hazards.
The Future of Cation Exchange
The cation exchange resin market is expected to grow at a compound annual growth rate of 5-6% through 2025, driven by increased demand from end-use industries like power, food & beverage, pharmaceutical, and others (References). Key trends shaping the future of cation exchange technology include:
Emerging applications in biopharmaceutical purification, rare earth metal recovery, radioactive waste treatment, and lithium extraction for batteries point to continued expansion of cation exchange uses [1]. Advances in resin chemistry and engineering will enable more selective separation and higher efficiency.
There is growing demand for eco-friendly, sustainable cation resins utilizing greener materials like bio-based polymers instead of fossil fuel-derived polymers [2]. Resin suppliers are developing greener production methods as well.
New technologies like simulated moving bed, continuous ion exchange, and membrane cascades allow for more efficient, automated, and continuous cation exchange processes versus traditional batch methods [3]. This drives higher throughput and productivity.
In summary, cation exchange resins will continue advancing in terms of application, sustainability, and process efficiency to meet growing demand across industries.

