Introduction to Cations
Cations are positively charged ions formed when an atom loses one or more electrons (Source: https://www.pinterest.com/pin/cation-definition-chemistry-cat-lover-tshirt-homecosi-in-2023–970385050946982104/). This loss of electrons results in the atom having more protons than electrons, giving it an overall positive charge. Cations form when atoms undergo processes like ionization, where electrons are removed from the atom. They can also form when atoms react and give up electrons to other species.
The charge on a cation is determined by the number of electrons lost. For example, if an atom loses one electron it will form a +1 charged cation. The purpose of cations is to balance the charges of anions in ionic compounds. Cations also play important roles in chemical reactions, conduction of electricity, and more.
Types of Cations
There are two main types of cations: main group cations and transition metal cations. Main group cations come from groups 1, 2, and 3 on the periodic table. Some examples include lithium (Li+), sodium (Na+), potassium (K+), magnesium (Mg2+), calcium (Ca2+), and aluminum (Al3+) [1]. These cations have a charge of +1, +2, or +3 since they lose electrons to attain a noble gas configuration.
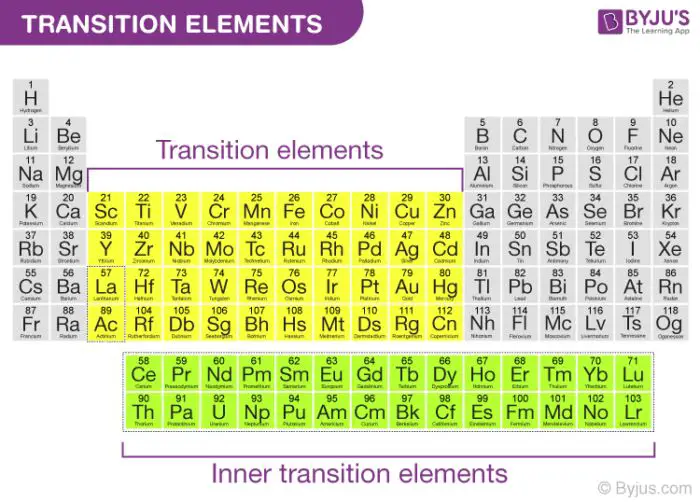
Transition metal cations are found in the d block of the periodic table. Examples include iron (Fe2+, Fe3+), copper (Cu2+), zinc (Zn2+), chromium (Cr3+), and manganese (Mn2+). They exhibit variable and multiple oxidation states. Transition metals form cations by losing electrons from their outer d orbitals [2]. The charge of transition metal cations depends on how many electrons are lost and can range from +1 to +7.
The key difference between main group and transition metal cations is that main group cations exhibit fixed, predictable charges based on their group number, while transition metals can form multiple cations with different charges. Additionally, transition metals lose d electrons whereas main group elements lose s or p electrons when forming cations.
Formation Through Ionization
Metals form cations through the process of ionization, which involves the metal atom losing electrons. Metals have relatively low ionization energy, meaning they can easily lose electrons to form cations with a positive charge. For example, sodium can lose its outermost electron to form the Na+ cation. This happens because metals have their valence electrons loosely held in their outer electron shell, so those electrons can be removed to expose the positive nuclear charge and create a cation. The number of electrons lost by a metal atom depends on its position on the periodic table and where it needs to gain stability. But in general, metals ionize by losing enough electrons to attain a full octet in their electron configuration, thereby forming a stable cation with a positive charge.

According to Quizlet, “How do metals form cations? By losing electrons enough to achieve a full octet.” The ionization process allows metals to give away their valence electrons and attain a cation’s stable electron configuration.
Formation through Accepting Electrons
Nonmetals tend to gain electrons to form anions. This is because nonmetals have relatively high electronegativity, meaning they have a strong attraction for electrons. By gaining electrons, nonmetals fill their valence electron shells and attain a stable noble gas electron configuration.
The electron affinity of an atom is a measure of its tendency to accept electrons. Atoms with high electron affinity readily accept electrons to form negative ions. Nonmetals generally have positive electron affinity values, meaning energy is released when they gain an electron. This makes it favorable for nonmetals to form anions.
For example, the electron affinity of chlorine is -349 kJ/mol. This means when chlorine accepts an electron to form a chloride ion (Cl-), 349 kJ of energy is released per mole of ions formed. The negative sign indicates energy is released in this process. So chlorine readily accepts an electron to form the chloride anion.
Other nonmetals with high electron affinity that readily form anions include oxygen, sulfur, selenium, and the halogens. Gaining electrons allows these nonmetals to fill their valence shell and achieve an octet configuration just like the noble gases. This stability provides the driving force for anion formation.
Cation Formation in Salts
Cations can form when acids react with bases in a process called neutralization. This reaction produces water and a salt as products. The salt contains the cation from the acid and the anion from the base. For example, when hydrochloric acid (HCl) reacts with sodium hydroxide (NaOH), the products are water and sodium chloride (NaCl). HCl provides the Cl- anion and NaOH provides the Na+ cation to form the NaCl salt.
In general, salts are formed when the hydrogen ion (H+) from an acid reacts with the hydroxide ion (OH-) from a base. The H+ loses its hydrogen and becomes a cation, while the OH- gains the hydrogen to become water (H2O). The sodium ion (Na+) does not change. This demonstrates how cations like Na+ form during salt formation.
Some other examples of cation formation during salt synthesis include:
– Calcium nitrate (Ca(NO3)2) forms from the reaction of calcium hydroxide (Ca(OH)2) and nitric acid (HNO3). The Ca2+ cation comes from the calcium hydroxide.
– Potassium chloride (KCl) forms from the reaction of potassium hydroxide (KOH) and hydrochloric acid (HCl). The K+ cation comes from the potassium hydroxide.
– Ammonium sulfate ((NH4)2SO4) forms from the reaction of ammonium hydroxide (NH4OH) and sulfuric acid (H2SO4). The NH4+ cation comes from the ammonium hydroxide.
In summary, cations form during acid-base neutralization reactions that produce salts. The metal cation originates from the base, while the nonmetal anion comes from the acid.
Cation Formation in Complexes
Complex ions, sometimes called coordination complexes, form when ligands donate electron pairs to a central metal cation. According to the LibreTexts source, a ligand can be a molecule or ion that contains at least one donor atom such as nitrogen, oxygen or sulfur that has at least one lone pair of electrons that can be shared with the metal ion.
When the ligand donates its lone pairs of electrons to the metal cation, a coordinate covalent bond is formed between the ligand and the metal. This coordinate bonding results in the formation of a complex ion with the metal at the center surrounded by the attached ligands. Some common ligands that form complex ions with metal cations include water, ammonia, chloride, cyanide, and ethylenediamine.
For example, copper(II) can react with four ammonia molecules to form the complex ion tetraaminecopper(II). In this complex, each ammonia molecule donates its lone pair of electrons to the copper(II) ion, resulting in a coordination number of 4 for the copper ion. The overall charge of the complex is still 2+ since the copper ion has a 2+ charge that is not changed by the attachment of the neutral ammonia ligands.
According to the LibreTexts source, the formation of complex ions allows metals to achieve coordination numbers between 2-8, whereas simple metal ions are generally limited to coordination numbers of 2, 4, or 6.
Properties of Cations
Cations have some key properties that determine their chemical behavior (Google Books, 2022):
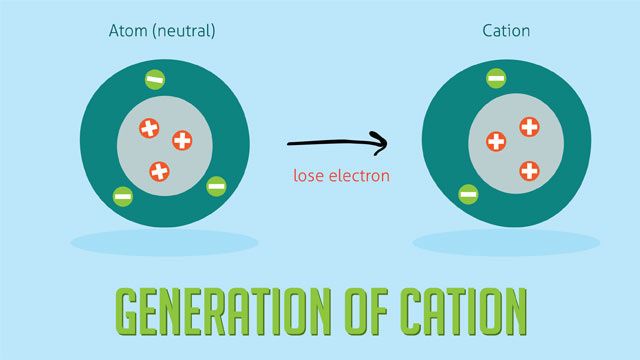
Charge: The charge on a cation is a positive charge, typically +1 or +2. This positive charge occurs because cations have lost electrons during ionization. Charge affects the cation’s reactivity and attractions to anions or ligands.
Size: The size of a cation relates to its ionic radius, which decreases across a period and increases down a group on the periodic table. Larger cations are easier to polarize and hydrate. Size impacts solubility and coordination chemistry.
Electronegativity: Electronegativity indicates the attraction for electrons. Cations have lower electronegativity than their parent atoms since they have lost electrons. This affects cation reactivity and bonding behavior.
Hydration Enthalpy: The hydration enthalpy is the energy change associated with a cation attaching water molecules. Highly charged small cations have the highest hydration enthalpies. Hydration impacts solubility and aqueous interactions.
These key cationic properties of charge, size, electronegativity, and hydration enthalpy determine the chemical behaviors of cations in solutions, complexes, salts, and other compounds.
Uses and Applications
Cations have many important uses and applications in everyday life. Some of the most notable uses are:
Batteries – Cations like lithium, sodium, and potassium are used in batteries. Lithium-ion batteries contain lithium cations and are commonly used in consumer electronics. The flow of cations allows batteries to store and release energy.
Fertilizers – Cations like ammonium (NH4+), potassium (K+), calcium (Ca2+), and magnesium (Mg2+) are essential components of fertilizers. These positively charged ions provide nutrients that are critical for plant growth.
Water treatment – Cations can help remove harmful contaminants from water through ion exchange. Positively charged resin beads exchange harmless cations for heavy metal cations like lead and copper, filtering them from water.
Food – Sodium, potassium, and calcium cations are important for nutrition and play key roles in various biological processes. Table salt consists of sodium and chloride ions.
Medicine – Magnesium cations help regulate muscle and nerve function. Lithium cations are used to treat bipolar disorder. Isotopes of radioactive cations aid in imaging and treating diseases.
Consumer goods – Detergents rely on surfactants containing charged cation heads that interact with water molecules and dirt. Cations in dyes and pigments provide desired colors.
Cation Stability
Cation stability refers to how readily a cation forms. There are several factors that affect the stability of cations:
Charge density – Cations with smaller sizes and higher charges have greater charge density, making them less stable and harder to form. For example, Li+ is harder to form than K+ due to its smaller size.
Polarizability – Elements that are more polarizable can stabilize charge better. The outer electrons in larger atoms can more easily distort to counteract the positive charge of the cation. So larger cations like Cs+ are more stable than smaller ones like Li+.
Hydration energy – The energy released when a cation interacts with water molecules also impacts stability. Small highly charged cations have greater hydration energies and are harder to dehydrate and form. Larger cations with lower charge have lower hydration energies and are easier to form.
Overall, the most stable cations are those with low charge density, high polarizability, and low hydration energy. The hardest cations to form are those with high charge density, low polarizability, and high hydration energy like Li+ and Mg2+. The easiest are those like Cs+ and Rb+ which have the opposite properties.
Understanding cation stability trends allows prediction of reactivity and aids synthesis of desired ionic compounds.
Quiz Yourself
Here are some key multiple choice questions to recap your knowledge on how cations form:
1. Which of the following describes how a cation forms through ionization?
A. An atom gains electrons
B. An atom loses electrons
C. A molecule breaks apart into ions
D. A molecule gains protons
The correct answer is B. An atom loses electrons to become a positively charged ion known as a cation.
2. What charge will a cation formed from a sodium atom most likely have?
A. +1
B. +2
C. -1
D. -2
The correct answer is A. Sodium commonly forms a +1 cation when it loses its single valence electron.
3. How can transition metals like copper form cations with multiple possible charges?
A. By gaining electrons
B. By losing varying numbers of electrons
C. By sharing electrons
D. By becoming anions
The correct answer is B. Transition metals can form cations with charges like +1, +2 etc. depending on how many electrons they lose during ionization.
Check your understanding of how cations form by testing yourself with more quiz questions!

